From french molécule, from modern latin molecula, diminutive of latin moles ‘mass’. A molecule is a group of two or more atoms bonded together, representing the smallest fundamental unit of a chemical compound that retains the chemical properties of that compound A molecule is an electrically neutral group of atoms held together by chemical bonds
Definition and Examples of a Molecule
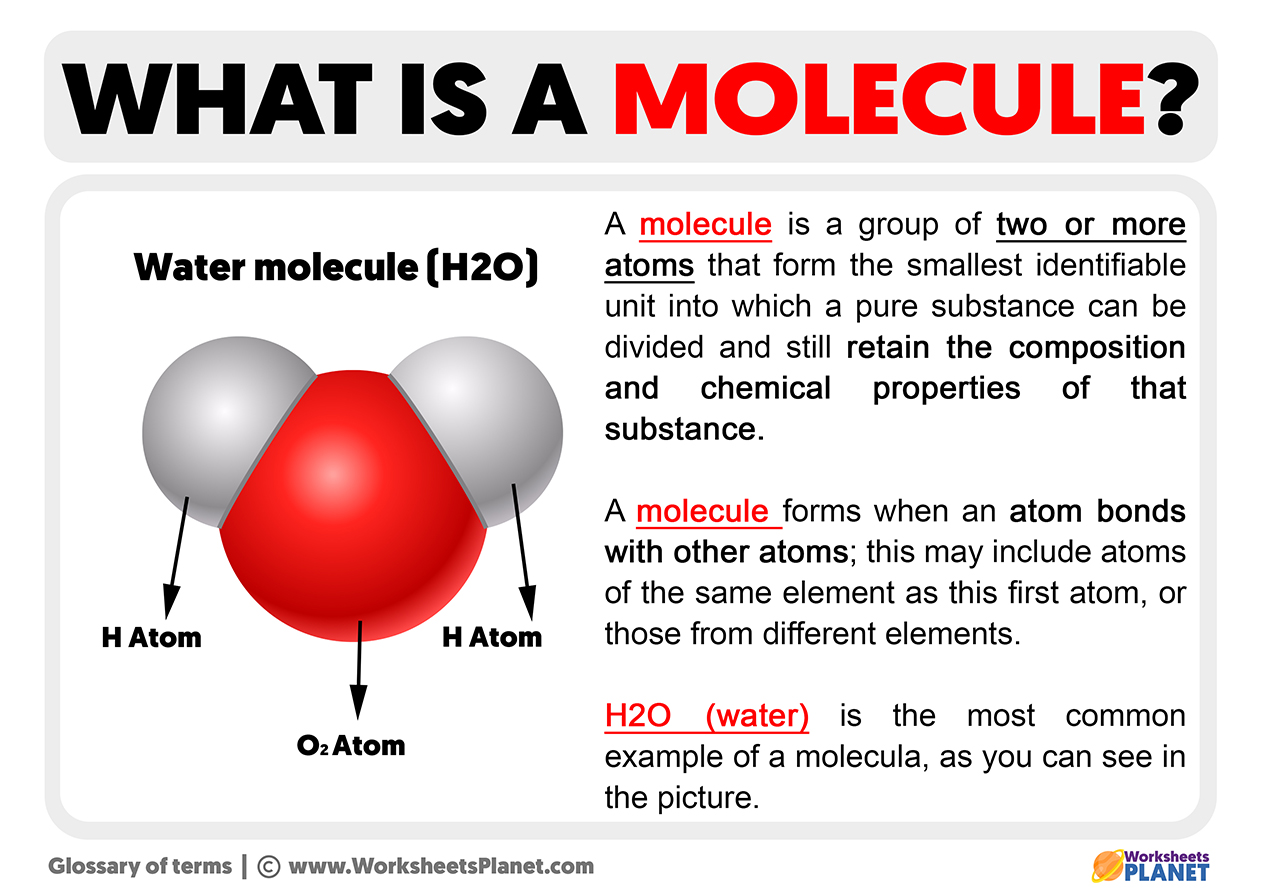
Learn the types, properties, and examples of molecules, and how they differ from compounds and ions. Molecules are made up of groups of atoms Molecule, a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition and chemical properties of that substance
Learn more about the properties and structures of molecules in this article.
The definition of the molecule has evolved as knowledge of the structure of molecules has increased Earlier definitions were less precise, defining molecules as the smallest particles of pure chemical substances that still retain their composition and chemical properties A molecule is the smallest particle of a substance that retains all the properties of the substance and is composed of one or more atoms A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound
Learn the difference between molecules and compounds, and see examples of common molecules such as water, nitrogen, and glucose. Learn the difference between atoms and molecules, and how they are the building blocks of matter Find out how to draw and name molecules, and see examples of elements and compounds. A molecule is two or more atoms bonded together to form a single chemical entity
Learn about the different types of molecules, such as proteins, lipids, carbohydrates, and nucleic acids, and their functions in biology.
The simplest unit of a chemical substance, usually a group of two or more atoms 2 Two or more atoms (same or different) that are held together by attractive forces or chemical bonds form a molecule The smallest unit of chemical elements having discrete chemical properties is the atom however, the characteristic of a matter depends upon the bonding between the atoms. The smallest unit of a chemical substance that can exist independently, consisting of one or more atoms bonded together
A substance made up of atoms of two or more different elements joined by chemical bonds Can be composed of one or more atoms of the same element or different elements. A molecule is a particle made up of two or more atoms that are chemically bonded together The number of atomic nuclei making up a molecule is a determinate number

For example, hcl (g) is a molecule made of one hydrogen atom bonded to one chlorine atom.
Many compounds do not fit the strict definition of a molecule but are common in chemistry and everyday life Some examples of structures that are not molecular in nature are crystals, minerals and metals Noble gases are elements that do not have valence electrons and, therefore, do not need to form covalent bonds to become stable 2) a complete chemical unit
While a molecule is often thought of as consisting of more than one atom, this is not always true For instance, helium has only one atom per molecule The oxygen molecule (o2) contains two atoms, as do chlorine (cl2), hydrogen (h2) and nitrogen (n2) Some molecules are huge having a molecular weight in the millions.
/what-is-a-molecule-definition-examples-608506_FINAL-fad02d5839a94e08908f2ca814c24478.png)
Modèle en 3 dimensions d'une molécule de saccharose
Schéma de la liaison covalente de deux atomes d'oxygène. Une molécule est une structure de base de la matière appartenant à la famille des composés covalents. See examples of molecule used in a sentence. The iupac compendium of chemical terminology
An electrically neutral entity consisting of more than one atom (\(n>1\)) Rigorously, a molecule, in which \(n>1\) must correspond to a depression on the potential energy surface that is deep enough to confine at least one vibrational state. A molecule is the smallest unit of a substance that has all the properties of that substance For instance, a water molecule is the smallest unit that is still water
/h20-58e655f93df78c5162ea0a1f.jpg)
A water molecule can be divided into tiny parts called atoms
This produces two hydrogen atoms and one oxygen atom But these atoms alone do not have the properties of water. A molecule is defined as the smallest unit of a compound that contains the chemical properties of the compound
Detail Author:
- Name : Dianna Rowe Sr.
- Username : mmueller
- Email : obuckridge@gmail.com
- Birthdate : 1987-05-14
- Address : 62439 Adelbert Square Apt. 192 Trudietown, GA 41085-7498
- Phone : +19707990852
- Company : Barton and Sons
- Job : Personal Service Worker
- Bio : Incidunt non qui accusamus officiis corporis. Maxime eos ducimus eos sapiente. Dolor hic assumenda quia sed tempore.
Socials
instagram:
- url : https://instagram.com/donavon6964
- username : donavon6964
- bio : Vitae ea culpa voluptas sed. Autem culpa ut rerum vitae. Culpa sunt quae voluptas.
- followers : 3645
- following : 2295
tiktok:
- url : https://tiktok.com/@donavon_real
- username : donavon_real
- bio : Corporis voluptatem quaerat minus vel excepturi.
- followers : 687
- following : 2513
linkedin:
- url : https://linkedin.com/in/donavon.rau
- username : donavon.rau
- bio : Et provident et qui.
- followers : 6315
- following : 2564
facebook:
- url : https://facebook.com/donavon_rau
- username : donavon_rau
- bio : Saepe suscipit reiciendis quis amet rerum. Velit est rerum magni.
- followers : 3985
- following : 2360
twitter:
- url : https://twitter.com/donavonrau
- username : donavonrau
- bio : Et eum ad totam consectetur. Consequatur voluptas laudantium eveniet.
- followers : 2765
- following : 2558